Infusion of memory T-cells in COVID19 disease

The severe acute respiratory syndrome caused by the coronavirus 2 (SARS-CoV-2) emerged in December 2019 causing a global pandemic. COVID-19 progression is generally biphasic, initially viral and afterwards inflammatory.
La Paz University Hospital of Madrid previously reported the existence of a SARS-CoV-2 specific T cell population within the CD45RA− memory T cells from the blood of convalescent donors. These cells can be stored and be immediately available as an “off-the-shelf” COVID-19 convalescent donor-derived biobank.
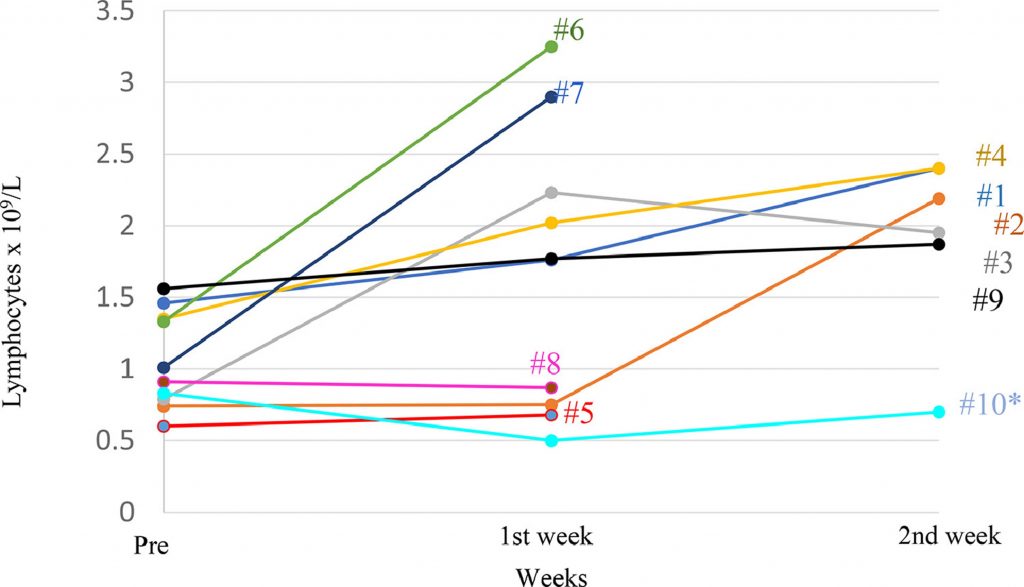
With the aim of determining the safety of a single infusion of memory T cells from a healthy donor recovered from COVID-19, evaluating time to lymphopenia recovery and to immune dysregulation, the time needed for a negative SARS-CoV-2 result by PCR, clinical improvement through the NEWS and a 7-category point ordinal scale, and length of stay, they set up a Phase I dose-escalation single centre clinical trial with 10 patients whose name was RELEASE and was published by The Lancet on August 12, 2021 with the collaboration of Geistek Pharmaceuticals.
 https://doi.org/10.1016/j.eclinm.2021.101086
https://doi.org/10.1016/j.eclinm.2021.101086
No adverse events including transfusion-related acute lung injury (TRALI), cytokine release syndrome (CRS), graft-versus-host disease (GvHD), fever, or sudden respiratory failure were observed. Therefore, DLT was not identified with doses up to 1 × 106 cells/Kg and the maximum tolerated doses (MTD) were not reached.
All participants sustained recovery after 28 days of follow-up, and no allogeneic toxic effect were reported using memory (CD45RA−) lymphocytes. No general adverse effects associated with the cryopreserved cell product in DMSO such as nausea, vomiting or abdominal cramps were observed.
They observed an improvement in clinical parameters measured by NEWS and 7-point scale two weeks post-infusion in both scales. Remarkable, patients with medium and high NEWS score at screening presented an improvement in the score to low risk and medium risk, respectively, at day 2 after infusion.
If you want to know more about this study, you can find it at https://doi.org/10.1016/j.eclinm.2021.101086
Infusion of memory T-cells in COVID19 disease
Infusion of memory T-cells in COVID19 disease
Share This Post
More To Explore

Tecartus
INTRODUCTION Tecartus is a new advanced therapy approved to treat adults with mantle cell lymphoma (MCL)¹. This disease is a lymphatic system cancer. Specifically, it

KYMRIAH, AN ANTI-CD19 CAR-T THERAPY
INTRODUCTION Kymriah (Novartis) is an advanced therapy medicine that belongs to the gene therapyproduct category. It is approved for B-cell acute lymphoblastic leukemia (ALL) in


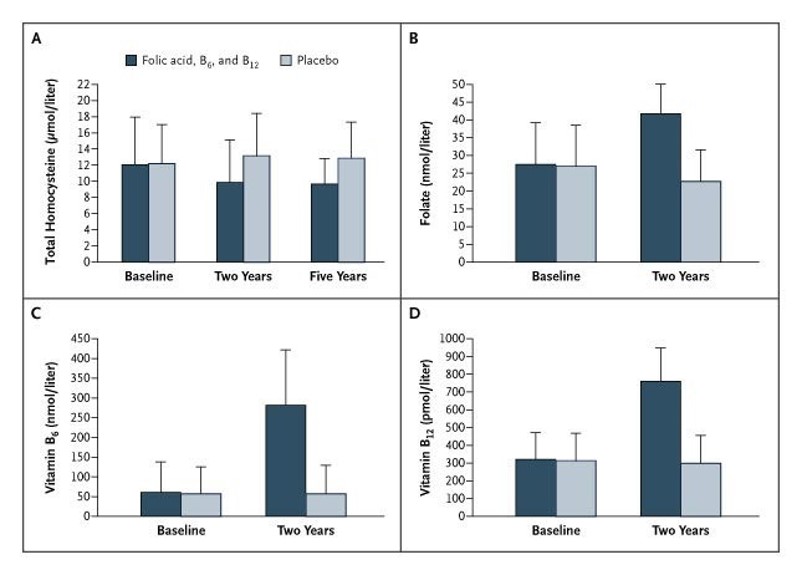 Effect of vitamins B6, B9 and B12 on homocysteine plasma decrease.
Effect of vitamins B6, B9 and B12 on homocysteine plasma decrease.