
Tecartus
INTRODUCTION Tecartus is a new advanced therapy approved to treat adults with mantle cell lymphoma (MCL)¹. This disease is a lymphatic system cancer. Specifically, it

Classic homocystinuria due to cystationine beta synthase deficiency is an autosomal recessive disease that affects 1 to 9 of every 100,000 newborns, but is detected in only 1 of every 344,000. In recent years, the incidence of this disease is rising notably, causing that in some areas of the planet it is found in 1 in every 20,000 children. This important increase is causing the number of studies that are carried out on this disease to be increasing notably. The first investigations published in PubMed date back to 1963, with 3 articles while, today, more than 80 research articles are published per year, the most recent being on the use of liver transplantation as an effective treatment in patients with classic homocystinuria , or on obsessive-compulsive behaviors in these patients as a pathognomonic manifestation.
The disease is diagnosed through 3 types of tests:
The characteristics that these patients present are:
The treatments that exist today for this pathology are of 2 types depending on the type of patient, and it is always recommended that it be diagnosed at birth:
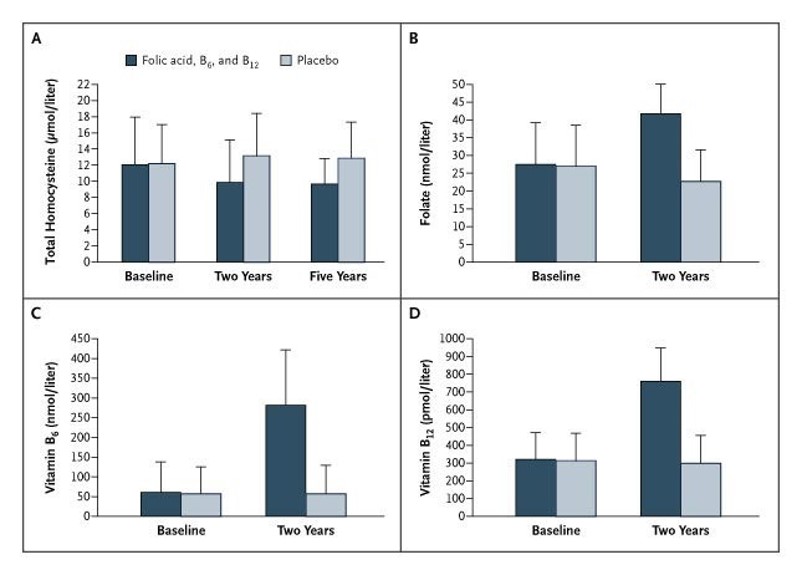 Effect of vitamins B6, B9 and B12 on homocysteine plasma decrease. https://www.nejm.org/doi/full/10.1056/nejmoa060900
Effect of vitamins B6, B9 and B12 on homocysteine plasma decrease. https://www.nejm.org/doi/full/10.1056/nejmoa060900
To date, there is only one clinical trial that is recruiting patients in the world. This is the NCT03406611 trial and it proposes using the drug OT-58 as a new system for the enzymatic replacement of CBS. The results are expected for December 2021, and through this blog, we will publish them so that families and patients can know as quickly as possible what the results have been.

INTRODUCTION Tecartus is a new advanced therapy approved to treat adults with mantle cell lymphoma (MCL)¹. This disease is a lymphatic system cancer. Specifically, it

INTRODUCTION Kymriah (Novartis) is an advanced therapy medicine that belongs to the gene therapyproduct category. It is approved for B-cell acute lymphoblastic leukemia (ALL) in
| Cookie | Duración | Descripción |
|---|---|---|
| cookielawinfo-checkbox-analytics | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Analytics". |
| cookielawinfo-checkbox-functional | 11 months | The cookie is set by GDPR cookie consent to record the user consent for the cookies in the category "Functional". |
| cookielawinfo-checkbox-necessary | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookies is used to store the user consent for the cookies in the category "Necessary". |
| cookielawinfo-checkbox-others | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Other. |
| cookielawinfo-checkbox-performance | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Performance". |
| viewed_cookie_policy | 11 months | The cookie is set by the GDPR Cookie Consent plugin and is used to store whether or not user has consented to the use of cookies. It does not store any personal data. |