
Uncategorized
Tecartus
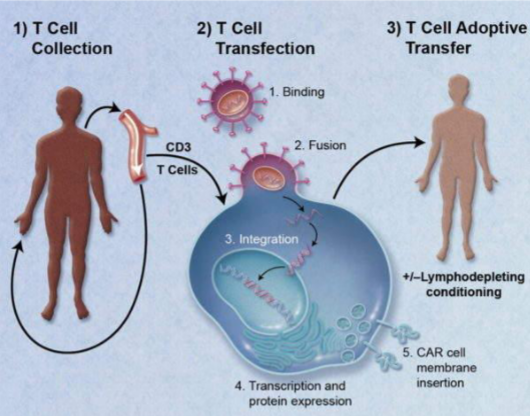
INTRODUCTION Tecartus is a new advanced therapy approved to treat adults with mantle cell lymphoma (MCL)¹. This disease is a lymphatic system cancer. Specifically, it

Need more information? Talk with us
Looking for project funding? Talk with us

INTRODUCTION Tecartus is a new advanced therapy approved to treat adults with mantle cell lymphoma (MCL)¹. This disease is a lymphatic system cancer. Specifically, it

INTRODUCTION Kymriah (Novartis) is an advanced therapy medicine that belongs to the gene therapyproduct category. It is approved for B-cell acute lymphoblastic leukemia (ALL) in
| Cookie | Duración | Descripción |
|---|---|---|
| cookielawinfo-checkbox-analytics | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Analytics". |
| cookielawinfo-checkbox-functional | 11 months | The cookie is set by GDPR cookie consent to record the user consent for the cookies in the category "Functional". |
| cookielawinfo-checkbox-necessary | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookies is used to store the user consent for the cookies in the category "Necessary". |
| cookielawinfo-checkbox-others | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Other. |
| cookielawinfo-checkbox-performance | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Performance". |
| viewed_cookie_policy | 11 months | The cookie is set by the GDPR Cookie Consent plugin and is used to store whether or not user has consented to the use of cookies. It does not store any personal data. |