Tecartus
INTRODUCTION
Tecartus is a new advanced therapy approved to treat adults with mantle cell lymphoma (MCL)¹. This disease is a lymphatic system cancer. Specifically, it is a type of B-cell non- Hodgkin’s lymphoma that develops from malignant B cells within the mantle zone, a region of the lymph node. 60–70-year-old men are the most frequent patients among the 200,000 individuals that are diagnosed with MCL each year. The most common first-line treatment is chemotherapy combined with immunotherapy, which can be accompanied by a stem cell transplant and/or an extended course of immunotherapy to prolong cancer remission. As mantle cell lymphoma is an uncommon form of non-Hodgkin’s lymphoma (NHL), accounting for 5% to 7% of all cases of NHL, it is considered a rare disease ². Therefore, Tecartus was considered an orphan medicine in November 2019. It is indicated after two or more unsuccessful treatments, including BTK inhibitors. Patients with mantle cell lymphoma have poor outcomes, particularly if the cancer is not responding to a BTK inhibitor or comes back after therapy with a BTK inhibitor. Tecartus provides a treatment option for these patients¹.
DRUG CHARACTERISTICS
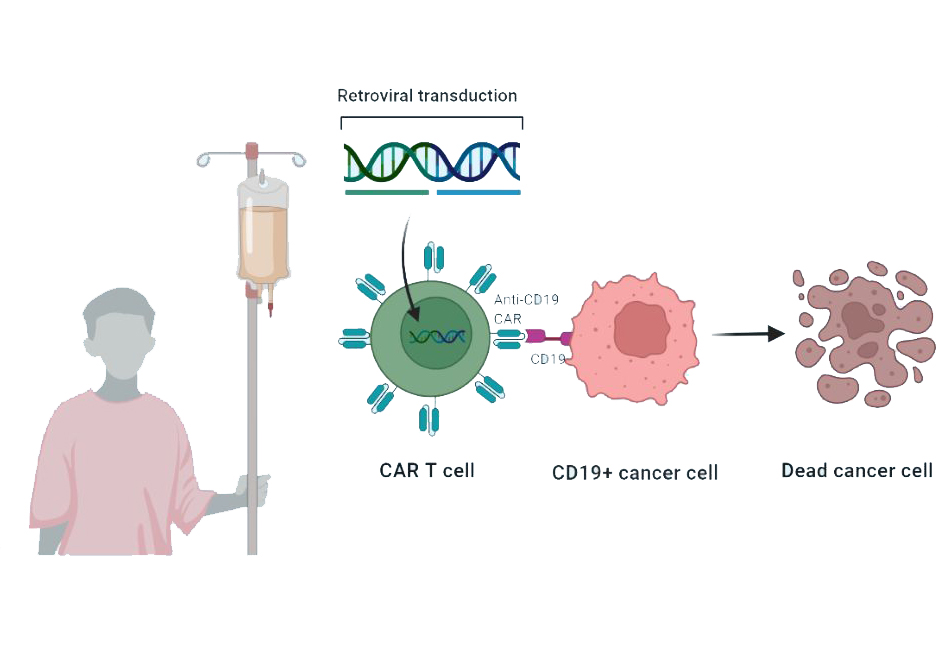
Autologous anti-CD19-transduced CD3+ T-cells make up the active substance of Tecartus. They are activated CD3+ T cells (including CD3+ CD4+ and CD3+ CD8+ cells) obtained from the patient blood, transduced with retroviral vectors expressing anti-CD19 CD28/CD3-zeta chimeric antigen receptor, and cultured to be reinfused. These cells can then attack cancer cells that express CD19. It is an advanced therapy englobed in CAR-T treatments, patient-specific, which is given as a single infusion into a vein after a short course of chemotherapy. Immediately before, the patient is treated with paracetamol and antihistamines to reduce the reactions to the infusion
APPROVAL
74 patients are currently involved in the main ongoing study, whose cancer has relapsed after at least two previous treatments including BTK inhibitors. Approximately, 59% of patients had a complete response, a higher percentage than with other treatments. Therefore, the EMA (European Medicines Agency) considered that Tecartus benefits are greater than its risks and they approved its use throughout the EU with a conditional authorization in December 2020¹.
RISK ASSESSMENT
More than half of the patients involved in the pivotal clinical study presented important side effects. Cytokine release syndrome is among them, being the most serious, as it is a potentially life-threatening condition that can cause pain, low blood pressure, shortness of breath, fever, and vomiting. Another severe side effect is encephalopathy, which is a brain disorder that causes headaches, mental confusion, and somnolence. Infections are also
common with this treatment. The risks are still being assessed, and there is still a lack of information about the long-term effects and safety in old patients, women, and with advanced disease. In case of cytokine release syndrome, the hospital must have available tocilizumab and emergency equipment. During at least ten days after treatment, patients should be closely monitored for side effects, and they should be close to a hospital for four weeks¹.

Need more information? Talk with us
Looking for project funding? Talk with us
REFERENCES
Tecartus
Tecartus
Share This Post
More To Explore

Uncategorized
Tecartus
INTRODUCTION Tecartus is a new advanced therapy approved to treat adults with mantle cell lymphoma (MCL)¹. This disease is a lymphatic system cancer. Specifically, it
Diego Fajardo
mayo 10, 2022

Uncategorized
KYMRIAH, AN ANTI-CD19 CAR-T THERAPY
INTRODUCTION Kymriah (Novartis) is an advanced therapy medicine that belongs to the gene therapyproduct category. It is approved for B-cell acute lymphoblastic leukemia (ALL) in
Diego Fajardo
abril 19, 2022